Magnesium is a promising alternative to lithium for cost-effective, high-capacity solid-state batteries, mainly due to its abundance, but the poor conductivity of magnesium ions (Mg2+) in solids at room temperatures has held back its practical application. Researchers at Tokyo University of Science (TUS) have addressed this decades-long roadblock by developing a novel Mg2+ conductor with a practically applicable superconductivity of 10-3 S cm-1.
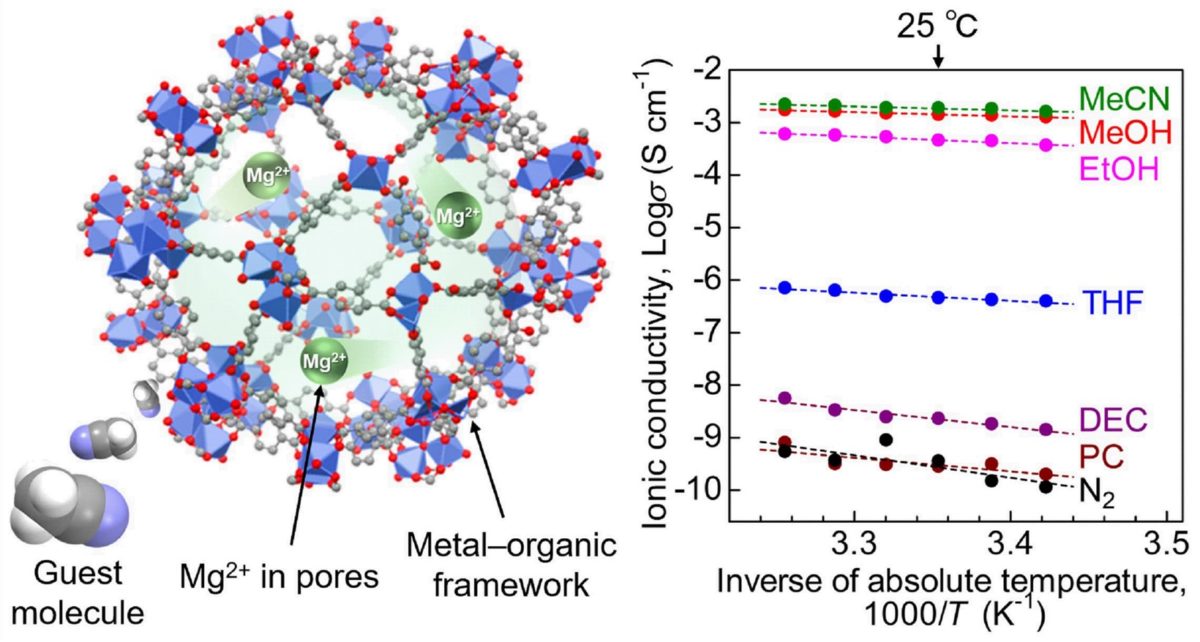
Mg2+ has poor solid-state conductivity because divalent positive ions (2+) experience strong interactions with their neighboring negative ions in a solid crystal, impeding their migration through the material. To address this, the researchers have exploited a class of materials called metal-organic frameworks (MOFs).
“MOFs have highly porous crystal structures, which provide the space for efficient migration of the included ions. Here, we additionally introduced a “guest molecule,” acetonitrile, into the pores of the MOF, which succeeded in strongly accelerating the conductivity of Mg2+,” said Masaaki Sadakiyo, the TUS junior associate professor who led the study.
The team used a MOF known as MIL-101 as the main framework and then encapsulated Mg2+ ions in its nanopores. In the resultant electrolyte, Mg2+ ions were loosely packed and could easily migrate. To further enhance ion conductivity, the research team exposed the electrolyte to acetonitrile vapors, which were adsorbed by the MOF as guest molecules.
The team then subjected the prepared samples to an alternating current (AC) impedance test to measure ionic conductivity. They found that the Mg2+ electrolyte exhibited a superionic conductivity of 1.9 × 10−3 S cm−1. The researchers believe this to be “the highest ever reported conductivity for a crystalline solid containing Mg2+.”
Popular content
To understand the mechanism behind this high conductivity, the researchers carried out infrared spectroscopic and adsorption isotherm measurements on the electrolyte. The tests revealed that the acetonitrile molecules adsorbed in the framework allowed for the efficient migration of the Mg2+ ions through the body of the solid electrolyte.
These findings, published in the Journal of the American Chemical Society, reveal the novel MOF-based Mg2+ conductor as a suitable material for battery applications. But they also provide critical insights into the development of solid-state batteries, which continue to attract interest and big investment from the automotive and energy storage industries.
“For a long time, people have believed that divalent or higher valency ions cannot be efficiently transferred through a solid. In this study, we have demonstrated that if the crystal structure and surrounding environment are well-designed, then a solid-state high-conductivity conductor is well within research,” explained Sadakiyo.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.


The superior technology of the solid state batteries with magnesium as a important conductor will enhance the advantages of electric vehicles. We congratulate the research team for their excellent achivement.