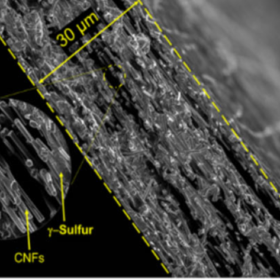
A 4,000 cycle lithium-sulfur battery
Scientists in the United States developed a lithium-sulfur battery using a commercially available carbonate electrolyte, that retained more than 80% of its initial capacity after 4000 cycles. The group used a vapor deposition process which unexpectedly produced a form of sulfur that did not react with the electrolyte, overcoming one of the key challenges for this battery chemistry.
Hold your breath for a better battery
Recent research has revealed a previously underestimated role for oxygen in limiting the performance of lithium-ion batteries. Newly published research from both Japan and the United States has sought to look deeper into the chemical reactions at the heart of lithium-ion storage; and to better characterize the cumulative effects that minuscule amounts of oxygen released during these reactions can have on battery performance and safety.
A long-lasting aluminum battery
Scientists in the U.S. developed an aluminum battery that demonstrates better than 99.5% reversibility, and could offer “up to 10,000 error-free cycles”. By incorporating a substrate of carbon fibers into the anode design, the group gained better control over chemical bonds that form as the battery charges, leading to greatly improved performance.
New method to produce silicon anodes for lithium-ion batteries
Scientists in Sweden developed a new aerogel process to manufacture silicon anodes for lithium-ion batteries, promising to offer batteries with greatly increased capacity compared to those on sale today. By growing nanometer-sized particles of silicon onto graphite, the group was able to demonstrate a device that overcomes many of the challenges common to silicon as anode material. While there are still challenges in terms of stability and capacity retention, the approach could ultimately yield low-cost, large-scale production processes.
Investigating moisture in nickel-rich batteries
Scientists in the UK looked into the effects of exposure to ambient atmosphere could have on nickel-manganese-cobalt cathodes for lithium-ion batteries. While many cathode designs are moisture sensitive, the group found that the nickel-rich cathodes currently gaining market share are especially vulnerable, and can suffer irreversible power loss upon exposure to moisture in the air.
Eliminate hydrogen for better battery performance, scientists say
Scientists at the University of California, Santa Barbara who are working with sodium-ion batteries have found that the unintended presence of hydrogen is to blame for many of the technology’s shortcomings in terms of degradation and performance loss. Keeping hydrogen out of the materials throughout production could allow sodium-ion batteries to achieve performance levels competing with their lithium-ion counterparts.