As the race for viable alternatives to lithium-ion technology accelerates, sodium batteries are emerging as one of the front-runners. Earlier this year, battery manufacturing heavyweight CATL unveiled plans to commercialize its first-generation sodium-ion batteries by 2023, bringing the technology out of the lab and into the real world of manufacturing.
Meanwhile, back in the labs, researchers are putting their heads together to solve some of the key hurdles that have held back sodium batteries from going mainstream. In a pair of new papers, two research groups at The University of Texas at Austin have detailed different pathways they took to addressing the same problem.
One of the groups has created a new electrolyte that eliminates some of the common issues of sodium-sulfur batteries by preventing the sulfur from dissolving away. They have demonstrated that by tweaking the solvation structure of the electrolyte, a solid-electrolyte interphase rich in inorganic components can be realized at both the sulfur cathode and the Na anode.
This means that the intermediate compounds formed from sulfur remain in a “half-dissolved” state instead of dissolving in the liquid electrolyte and migrating between the two electrodes within the battery. This process, known as shuttling, can lead to material loss, degradation of components, and the growth of dendrites on the anode. Needle-like filaments called dendrites can in turn cause the battery to rapidly degrade, short circuit, and even catch fire or explode.
By stopping the dissolution of sulfur, the new electrolyte eliminates Na polysulfide (NaPS) shuttling and facilitates dendrite-free Na-metal plating and stripping. This enables a longer life cycle for the battery, showing a stable performance over 300 charge-discharge cycles with a capacity fade of as low as 0.10% per cycle.
The researchers say that scalability of this approach to pouch cells demonstrates its potential for practical viability. Their findings were published in the Journal of the American Chemical Society.
Faster charging
Popular content
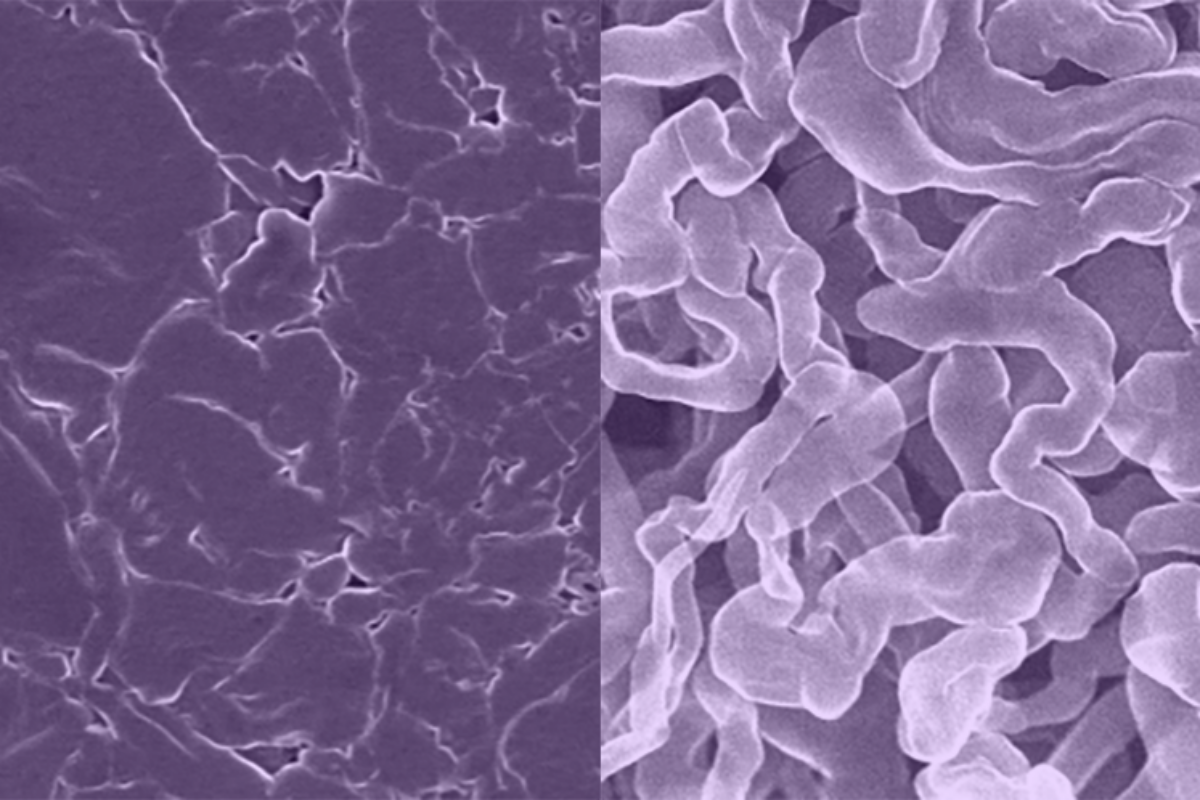
The other group or researchers has developed a new sodium metal anode for rechargeable batteries that resists the formation of dendrites. The new material, called sodium antimony telluride intermetallic – Na metal composite (NST-Na) – allows cycling at 100% depth of discharge and as an anode-free metal battery. Theoretically, it has the highest energy density of any sodium anode.
NST-Na is made by repeated cold rolling and folding, which involves rolling a thin sheet of sodium metal onto an antimony telluride powder, folding it over on itself, and repeating many times. The researchers found that this process leads to a uniform distribution of sodium atoms, which makes it less likely to form dendrites or surface corrosion.
Typically, the sodium atoms that carry a charge in a battery bind more strongly to each other than they do to the anode, and in so doing they tend to form instabilities, or clumps of sodium that attract more sodium atoms and eventually lead to dendrites.
Referring to their density functional theory calculations, the researchers say that the uniqueness of NST lies in the thermodynamic stability of the Na atoms (rather than clusters) on its surface that leads to planar wetting, and in its own stability that prevents decomposition during cycling. As a result, this makes the battery more stable and allows faster charging, comparable to a lithium-ion battery’s charge rate.
“We’re essentially solving two problems at once,” said David Mitlin, a professor in the Cockrell School of Engineering’s Walker Department of Mechanical Engineering and Applied Research Laboratory who designed the new material. “Typically, the faster you charge, the more of these dendrites you grow. So if you suppress dendrite growth, you can charge and discharge faster, because all of a sudden it’s safe.”
The scientists published their results in the journal Advanced Materials.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.


2 comments
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.