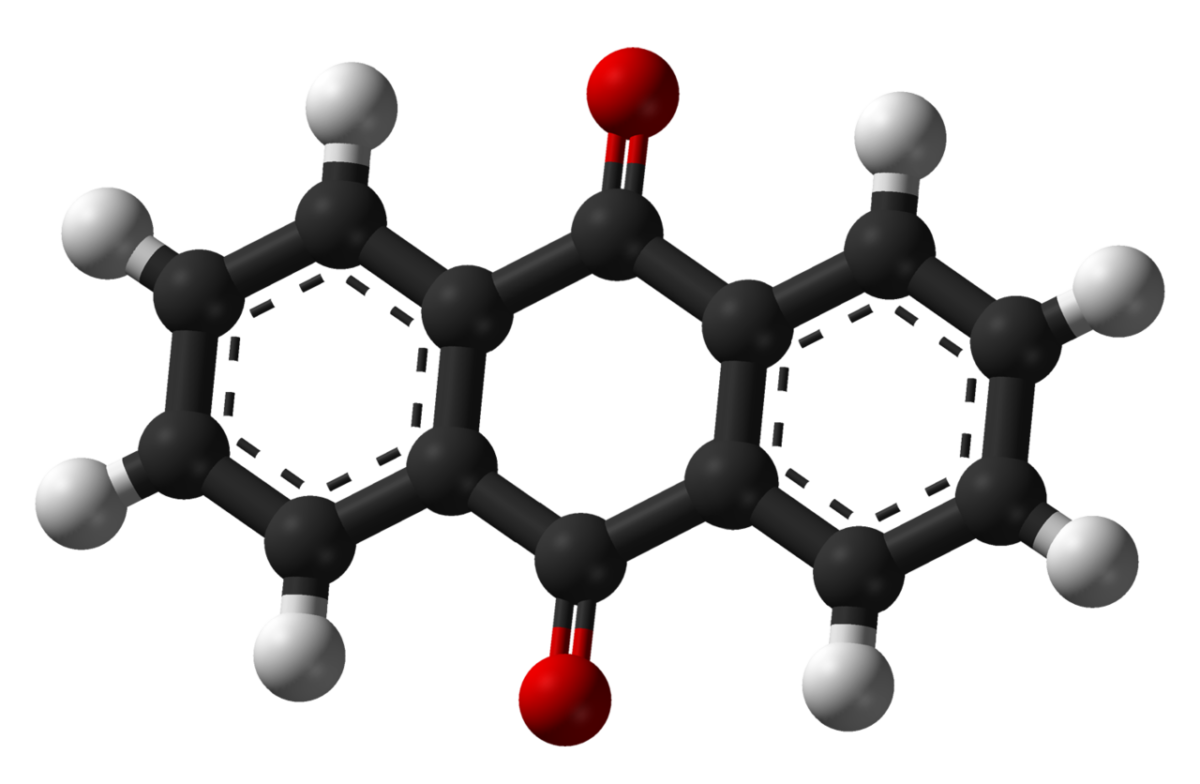
Scientists from the University of Cambridge and Harvard University claim to have considerably increased the duration of organic aqueous redox flow batteries. They used a molecule known as 2,6-dihydroxy-anthraquinone (DHAQ) – or more simply, anthraquinone – to avoid the decomposition of chemically unstable redox-active species, which is the main factor affecting the storage capacity of these storage devices.
“Organic aqueous redox flow batteries promise to significantly lower the costs of electricity storage from intermittent energy sources, but the instability of the organic molecules has hindered their commercialization,” said researcher Michael Aziz. “Now, we have a truly practical solution to extend the lifetime of these molecules, which is an enormous step to making these batteries competitive.”
DHAQ decomposes slowly over time, regardless of how many times battery cycles have been performed. When it is in contact with the air, after a cycle, this molecule absorbs oxygen and turns back into its original status. For this reason, the researchers refer to the molecule as a “zombie quinone,” as it is kind of returning to life after being dead.
“But regularly exposing a battery’s electrolyte to air isn’t exactly practical, as it drives the two sides of the battery out of balance – both sides of the battery can no longer be fully charged at the same time,” they said.
Through nuclear magnetic resonance (NMR), the academics discovered that the battery's active materials can be recomposed via deep discharge. This occurs in a battery when it has been discharged at its full capacity.
“Usually, in running batteries, you want to avoid draining the battery completely because it tends to degrade its components,” said researcher Yan Jing. “But we’ve found that this extreme discharge where we actually reverse the polarity can recompose these molecules – which was a surprise.”
Popular content
They said redox flow batteries developed via this approach could offer a net lifetime that is 17 times longer than previous research has shown.
“Getting to a single-digit percentage of loss per year is really enabling for widespread commercialization because it’s not a major financial burden to top off your tanks by a few percent each year,” said Aziz, adding that the proposed approach was applied with success to a range of organic molecules.
They described their findings in “In situ electrochemical recomposition of decomposed redox-active species in aqueous organic flow batteries,” which was recently published in Nature Chemistry.
“Now, utilizing 2,6-dihydroxy-anthraquinone (DHAQ) without further structural modification, we demonstrate that the regeneration of the original molecule after decomposition represents a viable route to achieve low-cost, long-lifetime aqueous organic redox flow batteries,” they said.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.


4 comments
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.