Scientists from the Oregon State University, in the United States, have proposed to improve the efficiency of compressed air energy storage (CAES) by recovering the thermochemical heat produced by facilities relying on the technology.
“Conventional CAES has relatively low cost compared to all kinds of batteries and we would imagine that this technology should only improve that advantage,” the research's corresponding author, Nicholas AuYeung, told pv magazine. “Cost analysis has not been performed yet, but we are interested in doing a thorough techno-economic analysis.”
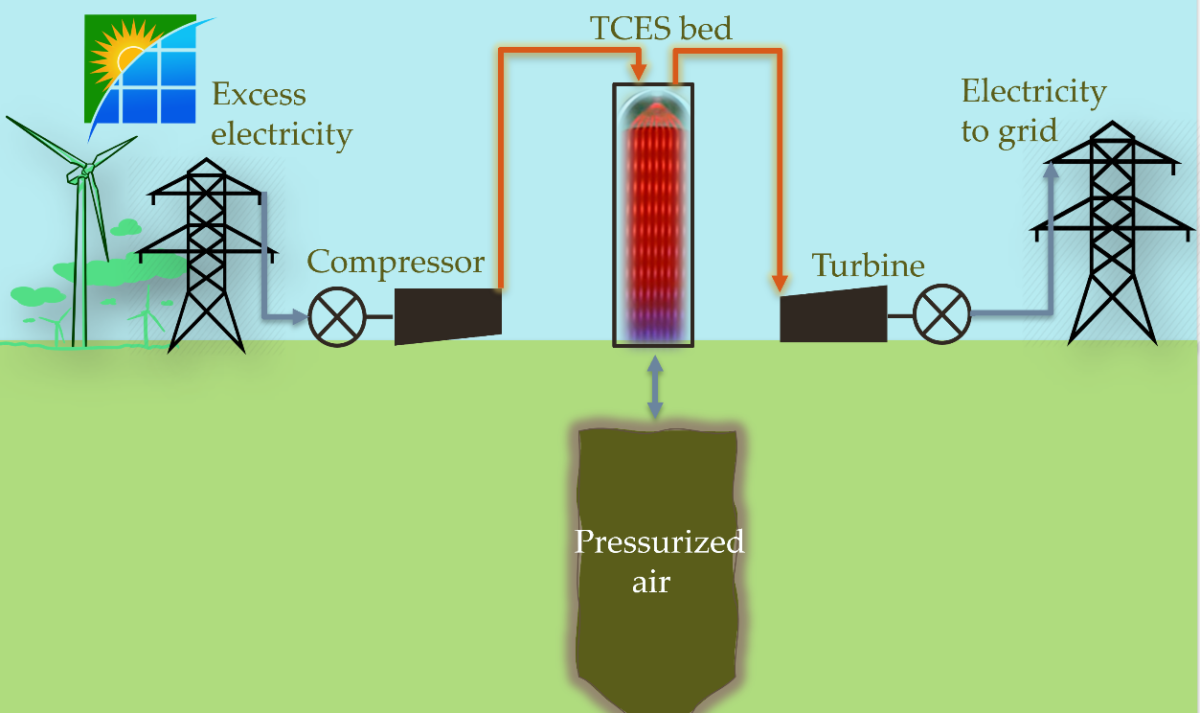
Described in the paper “Thermochemical heat recuperation for compressed air energy storage,” published in Energy Conversion and Management, the novel approach consists of applying a thermochemical energy storage (TCES) technique that stores energy in chemical bonds to recover the heat produced during air compression operations. “TCES systems based upon metal oxide redox reactions which can release oxygen under high partial pressures of oxygen are especially of interest, although any chemistry that can operate under high pressures could be considered,” the study reads. “These schemes typically take on the form of solid-gas reactions.”
The scientists proposed to use, in particular, resistance heating to decompose barium oxides in the charging step of CAES through three different strategies: Utilizing direct heat transfer through a reactive bed of TCES materials in a solid-gas reaction; indirect heat transfer between hot air and the TCES system; and a combination of direct and indirect heat transfer.
Popular content
“We looked at TCES with packed beds filled with rocks and barium oxides,” AuYeung stated. “Our results showed a similar round-trip efficiency between beds with TCES and beds without because of the relatively low heat capacity and heat of reaction for the barium oxides.”
According to him, the proposed system configuration can ensure a 60% round-trip efficiency with a 20-hour storage time after charge. This compares to a round-trip efficiency of only between 40% and 50% in conventional CAES. “To better illustrate the potential of the concept, we came up with a hypothetical material with the same heat capacity as rocks but a thermochemical storage capacity three times that of barium oxides, and we looked at that hypothetical material in our model,” AuYeung further explained. “Results showed that a potential round-trip efficiency improvement of more than 5% can be obtained, as well as longer storage duration. Also, 45% less filler volume would be needed to achieve storage capacity similar to rock-filled beds.”
Looking forward, the research team is planning to investigate more materials. “There are non-oxygen chemistries such as hydrates and carbonates that have the hypothetical properties – high heat capacity, high heat of reaction – we looked at, but right now we haven’t identified one for a redox material that operates on oxygen swing,” AuYeung concluded.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.


A marvellous example of blue-sky or or spherical-cow scientific optimism. The materials we have tested for TCES are no better than current CAES, so let’s assume much better ones!
All these Energy Storage Systems are not needed (just like polluting Fossil/Nuclear Energy) any l9nger.
S2S (Sunset-To-Sunrise) Energy is solved… to provide the 40,000 TWhrs/yr needed.. all by itself… to support an 180,000TWhrs/yr AgriVoltaics Global 150TW System that can meet ALL OF MANKINDS ENERGY NEEDS BY 2050… (9 BILLION PEOPLE USING 20,000KWhrs/petson/yr… or 180,000TWhrs/yr…. easily calculated)….
This Solution uses one of the Oldest, Reliable and Proven Technoligies too… and NOT REQUIRE ADDITIONAL RESOURCES EITHER…
Just Convert the 1TW Once-Thru / Open Loop to Multi-Use/Closed Loop UHES (Upgraded Hydro Energy Storage)… to generate 120TWHrs/Day (120×365= 43,800.. say 40,000TWhrs/yr).. by providing (new) 10TW of Pump-Motor Generators and only 120TWhrs (equivalent) of Water Storage in Lower Reservoirs (just 2.5% of the 5,000TWhrs/yr Storage of the existing Reservoirs above)… and operate in a daily 12/12 hr Pump/Generate Cycle… generating 120TWhrs/Day.
Check the Maths and you will see “THE TRUTH” GLARING AT YOU…
WITH A 150TW AV SYSTEM AND A 10TW UHES.. YOU GET SOLAR ELECTRICITY… DAY AND NIGHT.. 24 HOURS A DAY… POLLUTION FREE.. SUSTAINABLE (like existing Hydro Facilities) USING CURRENT TECHNOGIES TOO… ASAP…
The earlier this is done the sooner Mankind can end the Premature Deaths (9Million/yr) and Human Suffering (275Million DALY) that requires 2 Billion people/yr to seek Medical Intervention…
THIS IS THE REAL BENEFIT OF A GLOBAL ASAP POLICY FOR CONVERTING TO A ZERO POLLUTION WORLD… LESS DEATH AND SUFFERING…
EVERY DAY’S DELAY RESULTS IN 25,000 AVOIDABLE DEATHS AS WE JUST TALK.. TALK … TALK.. ON AND ON ON ( as for the last 50 Years )….