Scientists from the US Department of Energy's Pacific Northwest National Laboratory have developed an aqueous redox flow battery based on fluorenone derivative analytes.
The storage system is based on a low-cost organic compound known as fluorenone (C13H8O), which is largely used as a precursor to synthesize a variety of organic electronic materials and is bright fluorescent yellow in color. “Alternative materials for flow batteries include organic molecules, which are far more available, more environmentally friendly and less expensive than vanadium,” the US group stated. “But organics haven’t held up well to the demands of flow-battery technology, usually petering out faster than required.”
In order to prevent fast degradation, the fluorenone compound was transformed into a redox reversible, water-soluble compound. Prior to this technical improvement, fluorenone molecules were not water-soluble enough and could not provide redox reversibility in aqueous solutions. The solubility of the compound is crucial in redox flow batteries and the academics claimed to have been able to increase that of fluorenone in water from almost 0 with pristine fluorenone up to 1.5 moles per liter.
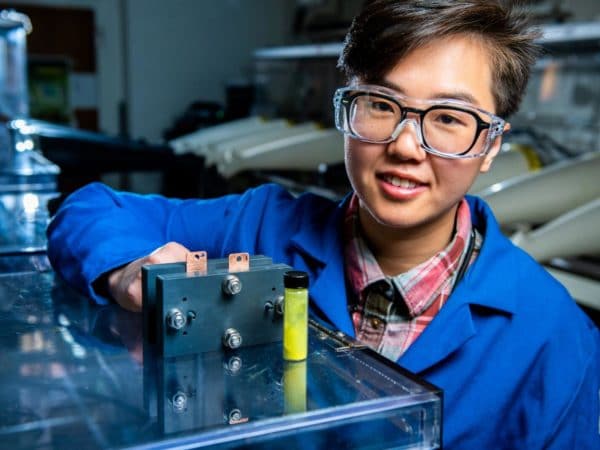
Image: Pacific Northwest National Laboratory
The research team found that the ability of fluorenone to carry out reversible reactions is dependent on its concentration. “This is a great demonstration of using molecular engineering to change a material from one widely considered impossible for use into something useful for energy storage,” said research co-author Wei Wang. “This opens up important new chemical space that we can explore.”
Popular content
The battery has a size of 10 cm2 and a power output of 500 milliwatts. Despite its small dimensions, its energy density is more than twice that of the vanadium batteries in use today, the scientists claim. “In laboratory testing that mimicked real-world conditions, the PNNL battery operated continuously for 120 days, ending only when other equipment unrelated to the battery itself wore out,” they further explained. “The battery went through 1,111 full cycles of charging and discharging — the equivalent of several years of operation under normal circumstances — and lost less than 3 percent of its energy capacity.”
The battery was presented in the paper “Reversible ketone hydrogenation and dehydrogenation for aqueous organic redox flow batteries”, published in Science.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



3 comments
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.