A group of researchers from South Korea's Ulsan National Institute of Science and Technology (UNIST) has designed a PV-powered scalable photoelectrochemical (PEC) system to produce green hydrogen that reportedly achieves a solar-to-hydrogen (STH) efficiency of 9.8%.
“Our system is a first attempt for short-term demonstration, we plan to change the designs as per our new upcoming research,” the research's lead author, Dharmesh Hansora, told pv magazine. “The unique advantage of our system is integrating several components in a single PEC device to avoid extra use of PV components, which minimizes the system complexity and reduces the system cost.”
The system utilizes a photoanode consisting of two PV cells made of a perovskite material known as formamidinium lead triiodide (FAPbI3). The photoanode is encapsulated in a nickel (Ni) foil and an electrocatalyst based on Ni, iron (Fe), and hydroperoxide (OOH).
The solar cells used for the photoanode have a p-i-n structure and are based on a substrate made of glass and fluorine-doped tin oxide (FTO). They also rely on an electron transport layer (ETL) made of titanium dioxide (TiO2), the perovskite absorber, a spiro-OMeTAD hole-blocking layer, and a gold (Au) metal contact.
The team also applied silver (Ag) paste as an ohmic junction metal between the FAPbI3 layer, and a passivation layer made of a Ni metal foil with a thickness of 25 μm. The foil was used to completely block the permeation of the electrolyte.
The researchers then deposited nickel–iron oxyhydroxide (NiFeOOH) as an oxygen evolution reaction (OER) co-catalyst on the Ni foil by drop-casting Ni and Fe precursor solution. “This metal-encapsulated FAPbI3 photoanode records a photocurrent density of 22.8 mA cm−2 at 1.23 VRHE and shows excellent stability for 3 days under simulated 1-sun illumination,” they stressed, noting that its performance is comparable to that of PV devices based on organic and inorganic metal halide perovskite (PSK) materials.
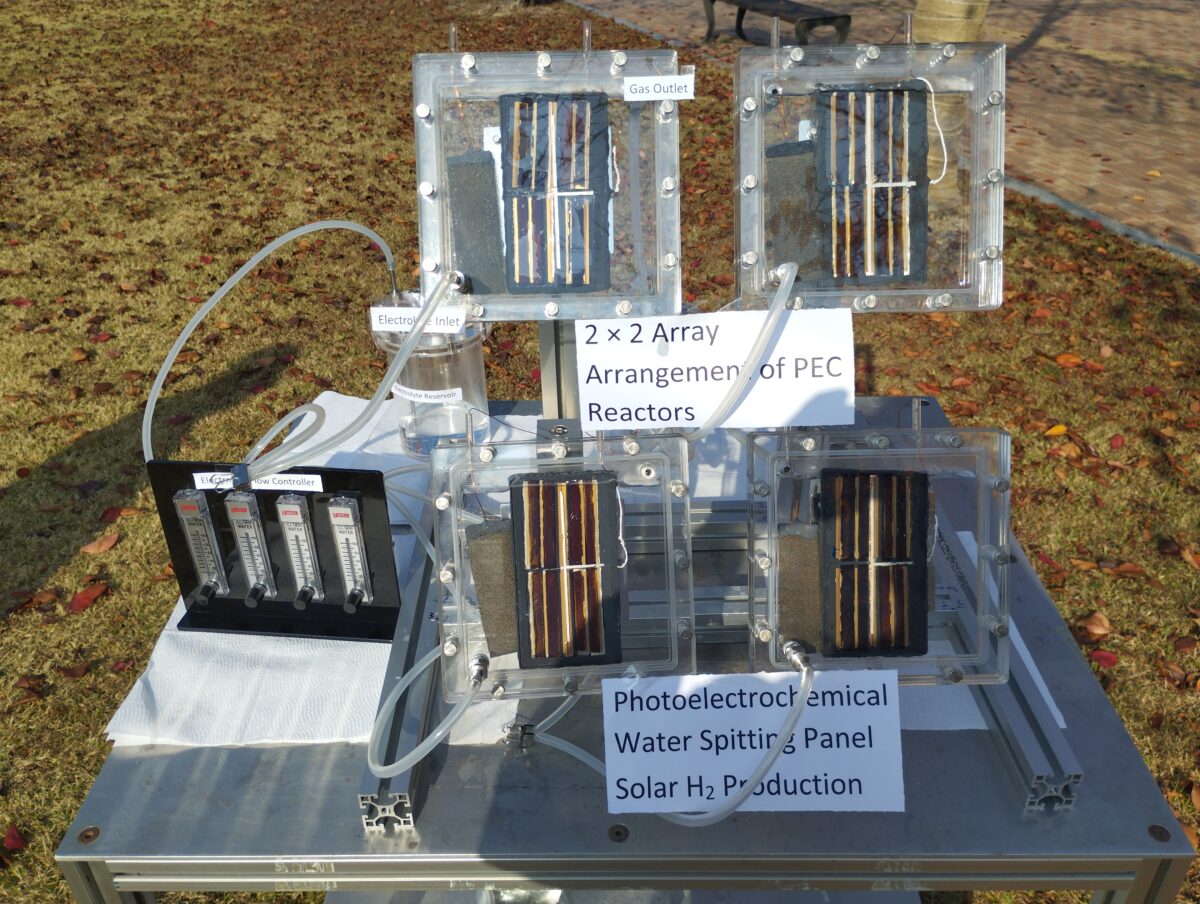
“We optimized this photoanode using different metal foil and studied the in-depth catalyst-electrolyte interactions,” Hanshora explained, noting that photoanodes with sizes of 0.25 cm2, 7.68 cm2, 30.8 cm2, and 123.2 cm2 were built and tested in a single reactor system.
Popular content
The research group then constructed the PEC water splitting system prototype by using the photoanode and a platinum (Pt) wire as a cathode. They immersed the anode and the cathode in the electrolyte inside the PEC reactor, while installing another FAPbI3 PV cell outside of the electrolyte in a parallel side-by-side connection.
“These small-area and scaled-up photoanodes recorded STH efficiencies of 9.8% (0.25 cm2), 8.9% (7.68 cm2), 8.5% (30.8 cm2) and 8.5% (123.2 cm2),” said the researchers, noting that photoanode was scaled up by increasing the unit cell size, the number of cells (multi-cell) and the number of reactors.
“Such results demonstrate the possibility that the high STH efficiency of small cells can be maintained in large-cell photoanodes,” they stated, while also acknowledging that the achieved efficiency values are still insufficient for practical PEC hydrogen production.
“We plan to improve further the efficiency and stability of the PEC system by integration of photoelectrodes and the selection of a more efficient and durable catalyst,” Hansora said.
The system was presented in the paper “All-perovskite-based unassisted photoelectrochemical water splitting system for efficient, stable and scalable solar hydrogen production,” which was recently published in nature energy.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.

1 comment
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.