Adding tin to the core of a germanium nanoparticle can help boost its light absorption properties, making such materials more efficient for photovoltaic applications, researchers at the U.S. Department of Energy’s Ames Laboratory have discovered.
The research team learned that the lattice structure of tin is a good match for the lattice structure of cadmium sulphide – the coating that is applied to nanoparticles to increase the amount of sunlight absorbed.
Germanium, used in such nanoparticles for solar cells, has very good electrical generation properties but does not absorb light too well – which is a critical component of the photovoltaic process.
The Ames researchers also found that the outside layer of germanium nanoparticles is prone to alteration over time as it oxidates. By applying surface passivation – essentially coating the nanoparticles with a light absorbing material – the photoluminescence capability of the nanoparticle improves.
By adding tin to the nanoparticles themselves, the germanium’s light absorption properties improved. A surface coating is still required, however, but the relationship – those lattice structures – between the atomic structure of the coating and the core material is better aligned, delivering a “more direct bandgap material”, explained Ames Lab scientist Emily Smith.
Popular content
The team has labelled this method Successive Ion Layer Absorption and Reaction (SILAR), having developed the technique over many years. “We hope to [via SILAR] continue making inroads in our ability to manipulate and direct energy flows at the nanoscale,” said fellow Ames scientist Javier Vela.
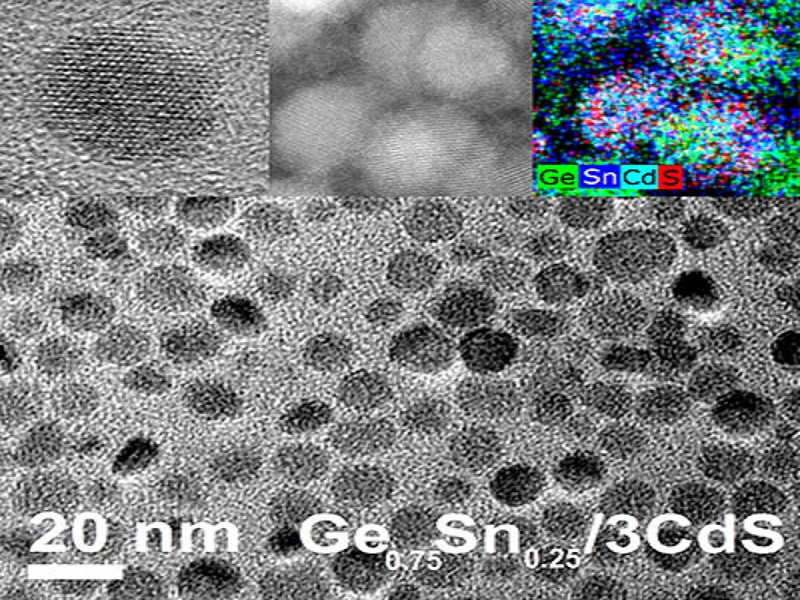
An advanced process of the technique involves using electron microscopy imaging and powder X-ray diffraction to better study the structural characteristics of the nanoparticles, allowing researchers to improve the correlation between the amount of tin required with the core’s lattice structure relation to the cadmium-sulfide outer shell.
“The atoms are in a very specific location within the nanocrystal core and when you apply the shell around the nanocrystal, the atoms of the shell may not match perfectly with the atoms of the core,” Smith said. “With the germanium only material used previously, the core and shell didn’t match perfectly.
“When we studied the germanium-tin particles, we proposed that they worked better because the spacing of the atoms better matches the spacing of the atoms we used in the coat layer,” she said. “By doing that, you’re getting a more perfect shell that’s less likely to cause chemical changes to the surface of the nanoparticle core.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.

By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.