The more amorphous a metal becomes, the better its electrochemical properties in a battery. However, the battery industry's poster child, lithium, appears mainly in crystalline form – leading to a host of issues with its behavior in lithium-ion batteries.
The production of amorphous metals is particularly challenging. However, a team of battery material researchers recently found a way to create amorphous metals, including lithium, in batteries – more or less by accident. The team from Idaho National Laboratories and the University of San Diego conducted research into what happens at the atomic level during the first few moments of recharging a lithium-ion battery.
The researchers discussed lithium's electrochemical reversibility in “Glassy Li metal anode for high-performance rechargeable Li batteries,” which was recently published in Nature Materials. This reversibility dictates lithium's morphology and its electrical properties. Hence, understanding this process better can lead to the development of higher-performance batteries.
Slow deposition
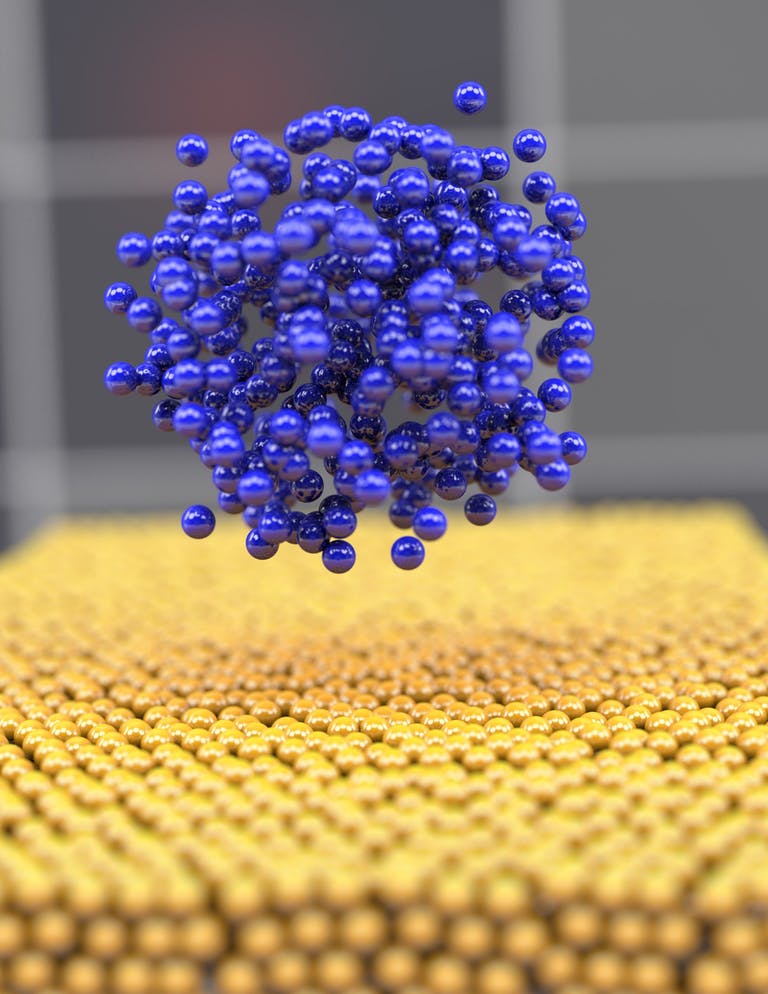
When cycling a battery, its lithium-ions deposit on the anode. The first stage of the process is known as nucleation, where the first tranche of metal-ions forms a starting point from which the rest of the crystalline metal grain grows. Using a cryogenic transmission electron microscopy, the team managed to visualize the initial lithium metal deposition on the anode for the first time. As lithium deposits in crystalline form, it is the primary nucleation that determines the way in which the remaining lithium grows around it. The researchers have dubbed this process “lithium embryo growth”.
“Depending on the atomic interaction during the initial nucleation (for example, the atom's packing density, mass transfer and energy transfer), the nanostructure of lithium nuclei can vary from disordered to ordered, which eventually shapes the final microstructure and affect the performance,” according to the Nature Materials article.
When lithium becomes too crystalline, it charges inconsistently, then subsequently leads to dendrite formation – the growth of irregularly shaped crystals. Such dendrites considerably shorten battery life.
“The power of cryogenic imaging to discover new phenomena in materials science is showcased in this work,” said Shirley Meng, who led UC San Diego's pioneering cryo-microscopy work. “It is true teamwork that enabled us to interpret the experimental data with confidence because the computational modeling helped decipher the complexity.”
Popular content
Much to the researchers' surprise, their experiments allows them to observe pure amorphous elemental metal for the first time. It also appeared that slow charging rates offer favorable conditions for amorphous lithium growth. Previously, the researchers had assumed that slower charging rates would lead to slower deposition rates, giving lithium-ions more time to find an ordered structure to arrange into, hence turning into unfavorable crystalline lithium.
Right environment
Aside from adjusting the charge current to achieve slower deposition rates, the researchers also used different electrolytes. In a “baseline” electrolyte, around 48% of the lithium assumed an amorphous state. But when applying what the researchers called an advanced electrolyte, 76% came out in an amorphous form. In both cases, the remainder formed crystalline structures.
According to the research, fiddling with the temporal and spatial confinements of mass and energy transfer with different strategies could produce glassy metal deposits. The strategies include methods to lower the current density, designing advanced electrolyte compositions, and employing 3D current collectors.
“These strategies are able to alter the bulk microstructure of a Li metal electrode and obtain larger, more homogeneous Li deposits with improved cycling performance,” the researchers said.
The research team has also identified other metals as potentially interesting for the battery industry. They include sodium, potassium, manganese, and zinc. Amorphous metals do not produce dendrites, so they can significantly improve the life cycle of batteries, in addition to their other improved electrochemical properties.
“These new amorphous active metals will open new opportunities in various applications besides the metallic glass and energy storage fields, including biomedicine, nanotechnology and micro- electromechanical systems,” the researchers concluded.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.


0 comments
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.