Aqueous batteries, that store energy in simple saltwater rather than complex organic solvents, are an attractive prospect for energy storage experts. These solvent-based electrolytes are often volatile and flammable and tend to degrade at high voltages. So replacing them with something as cheap, abundant, and fire-safe as seawater might seem like a no-brainer.
But this comes with its own set of problems. To date, aqueous battery technology has been held back by lower energy density compared to other battery technologies, as well as stability issues. Batteries with saltwater electrolytes are often prone to dendrite growth, and while this doesn’t come with a risk of fire as in standard lithium-ion technology it does still lead to performance loss and short-circuiting.
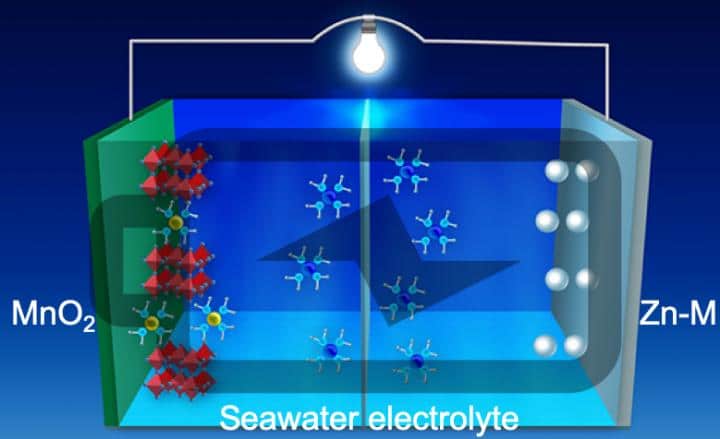
Scientists in the United States now claim to have overcome many of these issues using a new anode design. The group demonstrated this using a zinc-manganese alloy, though it says the approach could be extended to other metal alloys. “The use of the alloy with its special nanostructure not only suppresses dendrite formation by controlling the surface reaction thermodynamics and the reaction kinetics, said Zhenxing Feng, a chemical engineer at Oregon State University who worked on the research. “It also demonstrates super-high stability over thousands of cycles under harsh electrochemical conditions,”
Using these materials, with a 3D nanostructure on the anode’s surface, the group was able to much better control the reaction and avoid the formation of dendrites. To validate their approach, the group fabricated a battery and demonstrated stable, dendrite free cycling of the anode for 1,000 hours at a current density of 80mA/cm³. The work is described in the paper Stable, high-performance, dendrite free, seawater based aqueous batteries, published in Nature Communications.
Chemical observations
The paper also describes a new method developed by the group to observe in real-time the reactions happening at the anode, which should further improve their understanding of dendrite formation and other reactions taking place. This technique could also be applied to other battery concepts. “This platform provides us with the capability to directly image the electrode reaction dynamics in situ,” explained Xiaonan Shan, assistant professor of electrical and computer engineering at the University of Houston. “This important information provides direct evidence and visualization of the reaction kinetics and helps us to understand phenomena that could not be easily accessed previously.”
And though it’s a new discovery that will require a lot more testing to fully understand, the group has already begun to discuss the commercial potential of their approach. “Our theoretical and experimental studies proved that the 3D alloy anode has unprecedented interfacial stability, achieved by a favorable diffusion channel of zinc on the alloy surface,” Feng said. “The concept demonstrated in this collaborative work is likely to bring a paradigm shift in the design of high-performance alloy anodes for aqueous and non-aqueous batteries, revolutionizing the battery industry.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.