Lithium-rich cathode materials have been an area of interest for scientists working in energy storage since the early 2000s. In these materials, an oxygen-redox reaction has been shown to store additional charge in oxide ions, as well as in the transition metal ions – potentially boosting the material’s storage capacity.
However, when integrated into a battery, such cathode materials undergo irreversible structural changes upon the first charge, immediately reducing their subsequent voltage. And the mechanisms behind these structural changes have puzzled scientists and held the materials back from further development. With this in mind, the UK’s Faraday Institution set out to observe the structural changes to these cathodes in action.
“In the ever more difficult quest to make incremental improvements to Li-ion battery energy density, being able to harness the potential of oxygen-redox cathodes and the bigger improvements they offer relative to the nickel rich cathodes in commercial use today is potentially significant,” said Peter Bruce, Chief Scientist at the Faraday Institution. “The deeper understanding of the fundamental mechanisms of oxygen-redox is an important step in informing strategies to mitigate the current limitations of such materials, bringing their potential commercial use a step closer to reality.”
Oxygen oxidation
Using X-ray imaging techniques at the UK’s Diamond Light Source facility, the group was able to confirm the changes to oxygen that drive the voltage loss after the first charge, and also to develop a model that explains the entire process.
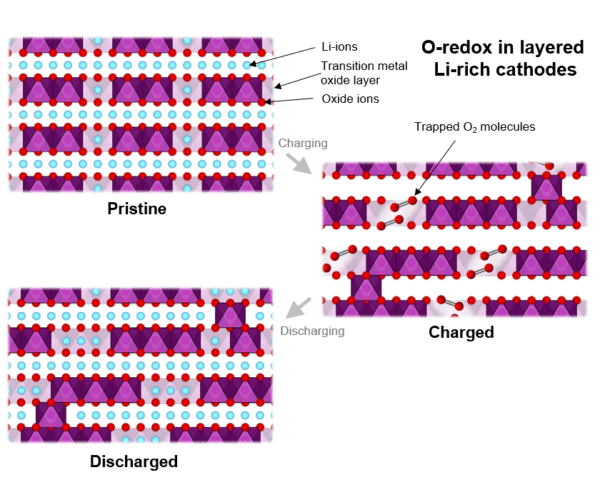
Image: Faraday Institution
“Computational modeling has demonstrated that the evolution of molecular oxygen explains both the observed electrochemical response – the reduction in voltage on first discharge – and the observed structural changes – explained by the accommodation of the molecular oxygen within the bulk of the material,” said Prof Saiful Islam, University of Bath and CATMAT Principal Investigator. “This single unified model tying molecular oxygen and voltage loss together allows researchers to propose practical strategies for avoiding oxygen-redox-induced instability, offering potential routes towards more reversible high energy density Li-ion cathodes.”
The model is described in the paper The role of O2 in O-redox cathodes for Li-ion batteries, published in Nature Energy. The researchers go on to suggest six different strategies to develop high-energy cathode materials based on this understanding of the oxygen-redox reaction, all of which are set to be explored by the Faraday Institution in follow-up work.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.




1 comment
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.