H2/H2O-power-to-gas-to-power systems that produce hydrogen by electrolysis and generate electricity from this hydrogen are promising large-scale electric energy storage systems for grids that are hosting ever greater share of intermittent renewables. On the flip side of the coin, such systems require complex thermal management and must be sufficiently large to improve the utilization of waste heat for higher system efficiency.
With this in mind, researchers from Japan’s Tokyo Institute of Technology (Tokyo Tech) are proposing an alternative system: a “carbon/air secondary battery” (CASB) that uses a carbon/carbon-dioxide redox reaction with potentially higher volumetric energy density and system efficiency than those of the hydrogen energy storage systems.
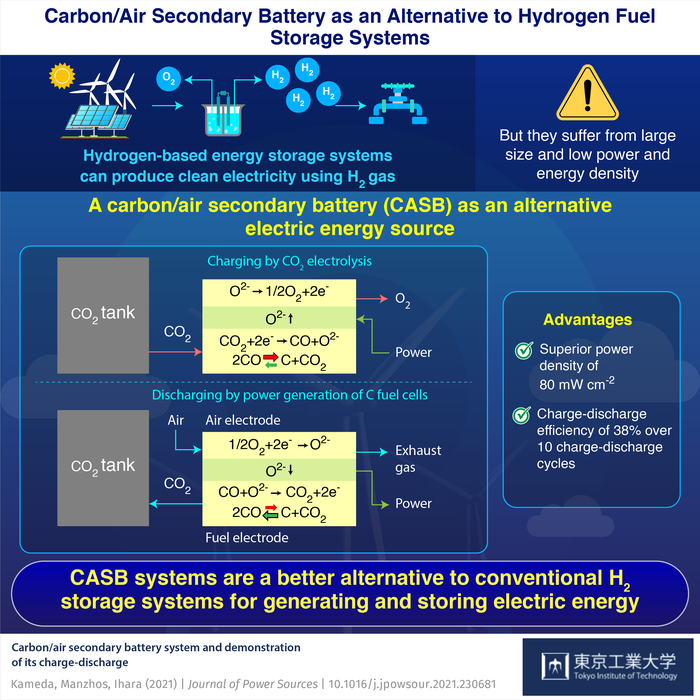
The CASB system is an electric energy storage system that consists of a solid-oxide fuel and electrolysis cell (SOFC/ECs) where carbon generated via electrolysis of carbon dioxide (CO2) is oxidized with air to produce energy. The SOFC/ECs can be supplied with compressed liquefied CO2 to make up the energy storage system.
“Similar to a battery, the CASB is charged using the energy generated by the renewable sources to reduce CO2 to C. During the subsequent discharge phase, the C is oxidized to generate energy,” explains Prof. Manabu Ihara of Tokyo Tech.
The researchers tested both the energy density and charge-discharge efficiency of the system. While the energy density of the CASB is limited by the amount of carbon it can hold, they found that the CASB had a higher volumetric energy density compared to hydrogen storage systems.
In addition, for the first time, repetitive power generation (10 charge-discharge cycles) of the CASB system with Boudouard equilibrium was demonstrated without degradation. Specifically, during the charging phase, C was deposited on the electrode via the electrochemical reduction of CO2 and the reduction of CO via the Boudouard decomposition. During the discharge phase, the C was oxidized to CO and CO2 via the Boudouard gasification reaction and electrochemical oxidation respectively. The researchers found that the C utilization for energy generation of the CASB depended on the equilibrium between the three different carbon species (C, CO2, CO), also known as the “Boudouard equilibrium.”
The CASB system was able to utilize most of the carbon deposited on the electrode for energy generation, achieving maximum Coulombic efficiency of 84%, indicating that most of the stored energy can be obtained during the discharge phase. Furthermore, it demonstrated charge-discharge efficiency of 38%, and power density of 80 mW cm−2 at 800° C and 100 mA cm−2.
“Compared to hydrogen storage systems, the CASB system is expected to have a smaller system size and higher system efficiency”, says Prof. Ihara. The findings were presented in the paper “Carbon/air secondary battery system and demonstration of its charge-discharge,” published in Journal of Power Sources.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



Why aren’t they storing the O2 and use it instead of air? At least the pure O2 can also be sold as a product.
Why is the label of the tank only showing CO2. In the Chemical reactions for recharging, CO is produced, so where is it stored? During electricity generation, CO is used, where did it come from?
Efficiency was an important ingredient when one was using/burning Fossil/Nuclwar Fuels for Energy…
Needless to say… the best energy is the one that is NOT USED at all… so efficiency still has a role but not so crucial when Pollution was created .. one way or another… by every Unit of Energy produced and consumed.
Today.. with the ability to convert abundant (unlimited..??), sustainable, non-polluting Solar Energy to Electricity directly using PV Panels the main issues are related to S2S (Sunset To Sunrise) Energy Storage and not creating pollution (all over again) by using it in Batteries or other devices or facilities..as the latter just “change the form” from one to another (air emissions to solid/liquid waste etc…)… and at present these “apparently clean” Batteries add to Pollution by using “dirty Energy” from the Grid with mostly Pollution causing Fossil/Nuclear Facilities too.. besides leaving behind toxic dead battery waste…
JUST USE … Clean, Abundant, Safe, Compressed Air… it is Non-Polluting, Cheap (no expensive batteries to replace… whose costs are “conveniently” forgotten by “experts and analysts” and spread by media as the infallible “word(s) of God” direct from the heavens above..)…
Apparent Efficiency maybe lower.. but with credit for “Free AirConditioning” and Energy Recovery during Air Compression, the Real Efficiency results in completly different conclusions)..