An international research team has developed a new concept for redox flow batteries that uses iron and chromium ore for redox chemistry.
“We are in the preliminary stages of exploring the economic aspect of these batteries,” the research's lead author, Hyun-Wook Lee, told pv magazine. “While we have not yet carried out a thorough levelized cost of storage (LCOS) estimation, our early findings point towards potential cost-competitiveness, especially given the environmentally friendly nature of our batteries and their promising performance metrics.”
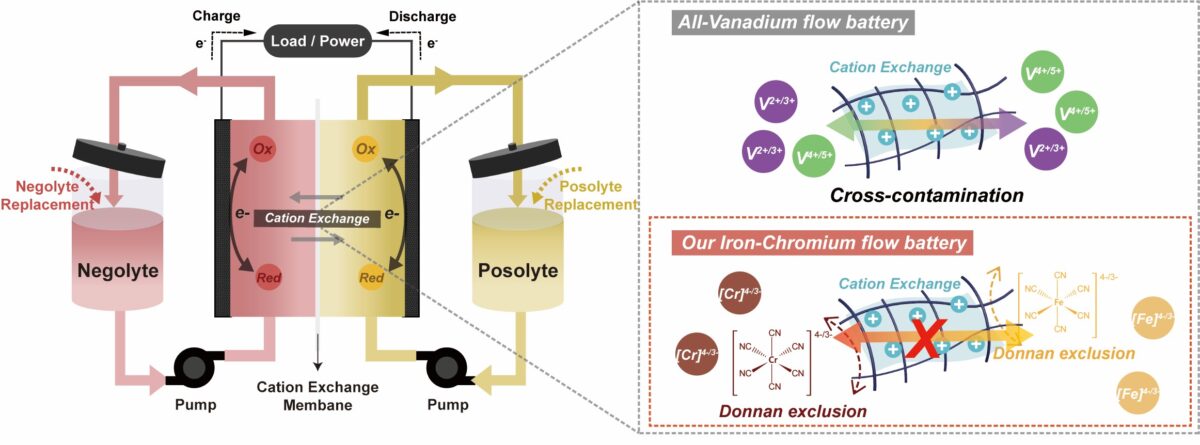
The battery relies on a chromium-based negative electrolyte, or negolyte, and strong-field cyanide ligands, which the scientists claim can mitigate the Jahn–Teller effect. The latter is a geometric distortion of a non-linear molecular system that reduces its symmetry and energy and, in redox flow batteries, reduces redox potential, stability, and rapid kinetics.
Suppressing the undesirable decomposition of the chromium(II) chloride Cr(II) complex used in the battery is the crucial step for avoiding these issues during the electrochemical cycling of redox flow batteries, thus facilitating a stable and fast redox reaction.
The battery also utilizes a negolyte based on hexacyanometalate, an inorganic compound with the formula Cr(CN)6. “The reliable electrochemical properties of Cr(CN)6 are unique because of its low redox potential of −1.15 V,” the group explained. The electrolyte was based on sodium cyanide (NaCN), which is claimed to offer excellent electrochemical stability, especially against very reductive potentials, combined with high solubility in water.
Popular content
Through a series of thermodynamic density-functional theory (DFT) simulations, the researchers found that the negolyte coordinates well with the strong-field ligands used for the battery and is able to reduce the Jahn–Teller effect.
The proposed battery configuration may achieve a stable lifetime of 500 cycles and a high-energy density of 38.6 Wh L−1, according to the research group.
“This hexacyanometalate-based redox flow battery is capable of reversible redox reactions with relatively high potential as an aqueous system taking advantage of the low potential of Cr(CN)6 and improved cycling performance at high current densities,” it stated. “In comparison to conventional and other flow batteries, this cell configuration has superior properties in terms of the full-cell potential and cycling performance.”
The new battery concept is presented in the study “Full-Hexacyanometallate Aqueous Redox Flow Batteries Exceeding 1.5 V in an Aqueous Solution,” published in Advanced Energy Materials. The research group includes scientists from the Nanyang Technological University in Singapore and the Ulsan National Institute of Science and Technology (UNIST) in South Korea.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.


By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.