A British research team from the University of Warwick and the Imperial College London is proposing the use of hybrid electrodes to bring redox flow batteries (RFBs) closer to commercial viability.
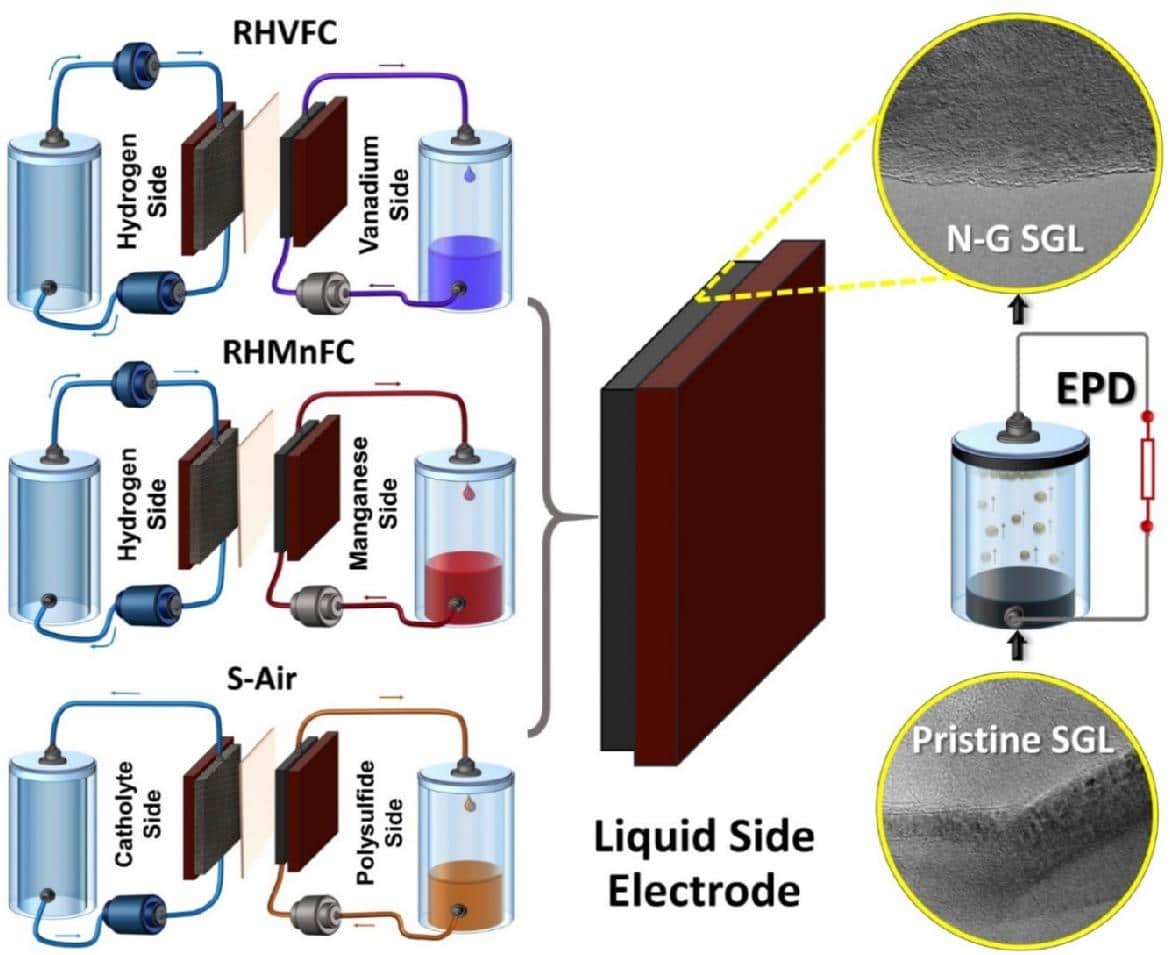
The scientists utilized standard carbon paper electrodes (CP) modified with electrophoretically deposited nitrogen-doped graphene (N-G), which they claim are capable of meeting the electrochemical requirements of the hybrid redox flow cells (HRFCs). These cells are key components for the development of reliable large-scale redox flow storage systems and usually consist of an electrochemical cell, a storage tank, and a hydrogen or oxygen gas cylinder.
The high cost of HRFCs, as well as low cycle performance, and incompatibilities associated with the commonly used carbon-based electrodes, however, have so far limited their commercial viability. “For cost-effectiveness and maximizing the benefits of RFBs and HRFCs, they need to be operated at higher current densities, as this can effectively reduce the size and number of cells per stack,” the researchers stated. “This basically depends on how efficiently HRFC electrodes are engineered in terms of their microstructural features and the extent to which they can cope with their harsh operating conditions.”
The academics applied the hybrid electrodes to three types of HRFCs: a hydrogen/vanadium (RHVFC) device; a hydrogen/manganese (RHMnFC) cell; and a polysulfide/air (S-Air) device. A binder-free horizontal electrophoretic deposition (EPD) technique was used to activate the electrodes. EPD is a two-step process enabling the particles suspended in a colloid solution to be collected onto a substrate. According to the scientists, this technique is a binderless process with precise control of few-layer graphene particles deposition with the exclusion of graphene aggregates. “Carbon paper (CP) acting as a gas diffusion layer (SGL 10AA, henceforth termed SGL) was used as the positive electrode for both the RHVFC and RHMnFC and as the negative electrode for S-A ir cells,” they explained.
Tests were conducted to compare the electrochemical performance of the three electrodes with that of commercially available liquid–liquid, all-vanadium RFBs. “We have demonstrated that these electrodes are capable of meeting the harsh electrochemical requirements for these HRFCs, evidenced by their enhanced performance and operability, attributed to the high specificity of these electrodes coming from the synergistic effects of enhanced electrochemical surface area, chemical/electrochemical stability, and catalytic activity,” the UK group concluded. “Furthermore, the estimated system cost shows that HRFCs are potentially low-cost alternatives to the commercial standard VRFB.”
Looking forward, the academics are planning to optimize the HRFCs by replacing conventional carbon electrodes with N-G-modified electrodes or free-standing hierarchical nanomaterial structures. Their findings were presented in the study Hybrid Redox Flow Cells with Enhanced Electrochemical Performance via Binderless and Electrophoretically Deposited Nitrogen-Doped Graphene on Carbon Paper Electrodes, published in ACS Publications.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.