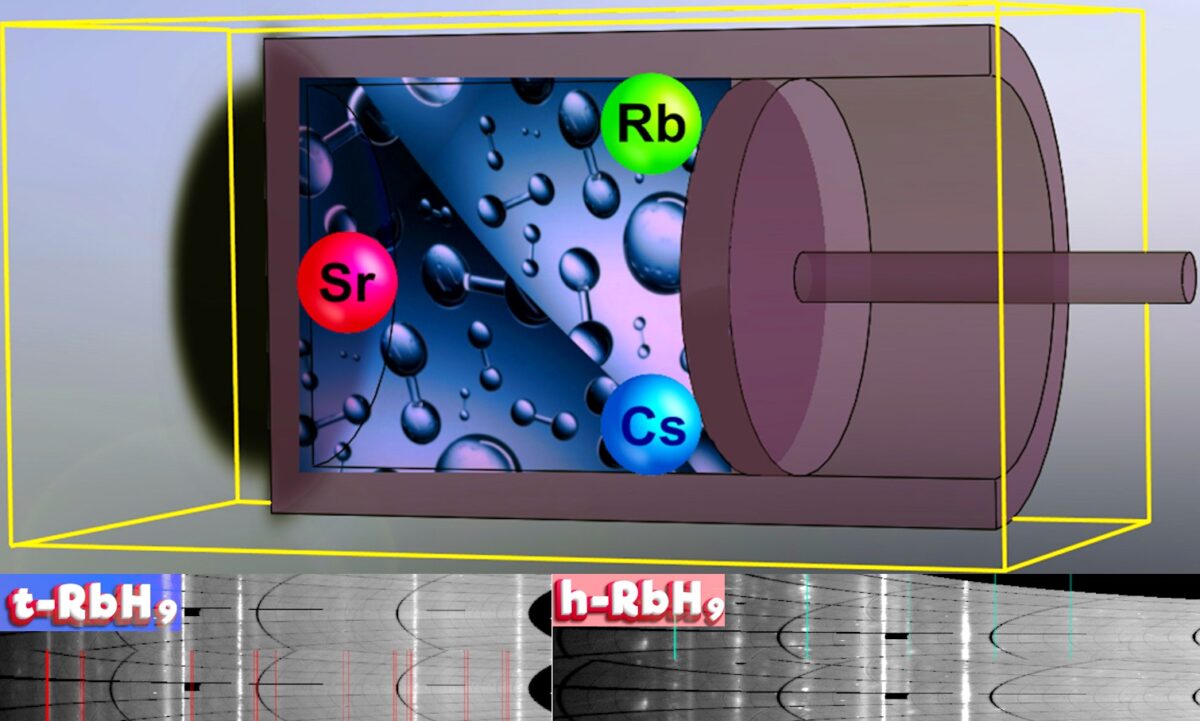
Russian, Chinese, Japanese, and Italian scientists have synthesized two polyhydrides – cesium heptahydride (CsH7) and rubidium nonahydride (RbH9) – for the chemical storage of hydrogen. They claim they “can absorb as much four times” hydrogen as frequently used magnesium-nickel and zirconium-vanadium alloys.
“Our work demonstrates that cesium and rubidium have prospects for increasing the capacity of hydrogen batteries by stabilizing the shell of molecular hydrogen, and open a way for further reducing the synthesis pressure of polyhydrides,” the authors wrote in “Raisins in a Hydrogen Pie: Ultrastable Cesium and Rubidium Polyhydrides,” which was recently published in Advanced Energy Materials.
Cesium (Cs) and rubidium (Rb) pack seven and nine hydrogen atoms per metal atom, respectively.
“The proportion of hydrogen atoms in these compounds is one of the highest among all known polyhydrides, twice as high as in methane CH4,” Dmitrii Semenok, a researcher at China’s Center for High Pressure Science and Technology Advanced Research (HPSTAR), told pv magazine.
Semenok noted that that Сs and Rb are relatively heavy atoms.
“For rubidium hydride RbH9 the mass content of hydrogen is about 9.6% wt,” he said. “This is also a very good result. The only problem is the need to apply pressure to synthesise these compounds.”
He explained that cesium and rubidium and their hydrides present two additional problems. Global production of Cs and Rb is significantly lower than that of other alkali metals, and the cost is substantially higher than lithium, sodium or potassium. They are also very active metals that easily catch fire in the air.
Popular content
The compounds are therefore not ideal for hydrogen storage, but the study led by Russia’s Skoltech and China’s HPSTAR suggests that rubidium and cesium additives can improve the efficiency of current hydrogen batteries.
“It is a question of changing the approach to the search for promising hydrogen storage materials,” said Semenok. “We work at much higher pressures than previously accepted in this field. And we use elements that tend to form higher polyhydrides at low pressures.”
He added that systems such as Strontium-Rubidium Sr-Rb-H, Strontium-Rb-Zirconium-H, Rb-Magnesium-H might be promising for hydrogen storage. They remain stable under ambient conditions after they are saturated with hydrogen at high pressure of several GPa or even lower.
“The formation of higher cesium and rubidium hydrides at a relatively low pressure of about 10 GPa was predicted theoretically more than 10 years ago in the works of the group of E. Zurek (UB). The problem was only in the experimental validation of these results,” Semenok said. “Since the discovery of sulfur trihydride (H3S), the main attention of experimentalists has been focused on superconducting hydrides.”
The situation has only recently changed due to the exhaustion of the class of binary superconducting hydrides. The researchers also proposed a new method for synthesis of metal polyhydrides via high-pressure thermal decomposition of corresponding amidoboranes in diamond anvil cells.
“We now intend to repeat the experiment using large-scale hydraulic presses at a lower pressure – about 10,000 atmospheres – to obtain larger amounts of cesium and rubidium polyhydrides and verify that once synthesized, these compounds remain stable even at atmospheric pressure, unlike the other polyhydrides known to date,” said Semenok. “This is an important task from a fundamental point of view. We need to answer the question how long metastable polyhydrides will exist under ambient conditions.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.

By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.