Blatter radicals are organic compounds that have remarkable electronic and magnetic properties, strong chemical and thermal stability and reversible redox properties. All these characteristics make them potentially suitable for several applications, including their use in redox flow batteries.
A Dutch-Danish research team has used these compounds, which are also known as 1,2,4-benzotriazin-4-yl radicals, to build a prototype of a symmetric redox flow battery for applications in stationary storage. “Blatter radicals combine tunable redox potentials and high stability in all relevant redox states necessary for application as redox-active components in redox flow batteries,” the academics stated.
The scientists selected a single molecule that is intrinsically stable and that can accept or donate electrons, which means it can be used on both sides of the battery and, at the same time, show how minimal capacity fade during battery cycling.
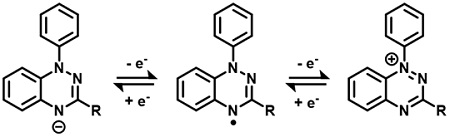
Image: E. Otten, University of Groningen
The special compound was used in a small electrochemical cell and was found to remain stable over 275 charge/discharge cycles. “We need to bring this up to thousands of cycles; however, our experiments are a proof of concept. It is possible to make a symmetrical flow battery that has good stability,” said Edwin Otten, Associate Professor of Molecular Inorganic Chemistry at the University of Groningen.
Popular content
“Another advantage of our symmetrical design is that it is not a big problem if some of our compound crosses the membrane during use,” explained Otten. “This could result in a slightly higher volume in one of the tanks but any imbalance is easily restored by simply reversing the polarity,”
A full description of the battery technology can be found in the study Blatter Radicals as Bipolar Materials for Symmetrical Redox-Flow Batteries, published in ACS Publications. The research group includes scientists from the University of Groningen and the University of Eindhoven in the Netherlands, as well as from the Technical University of Denmark. “We have shown that 1,2,4-benzotriazin-4-yl radicals combine bipolar electrochemistry with high chemical stability in all states of charge, which renders these compounds promising energy-storage materials for symmetric redox-flow batteries,” it concluded.
Looking forward, the academics are planning to develop a water-soluble version of the Blatter radicals.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.


By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.