While lithium metal batteries could offer better safety, better energy density, and lower weight than lithium-ion technology – thanks to the replacement of heavier graphite with lithium metal as anode – this battery chemistry does not work well with conventional electrolytes.
Therefore, research efforts have been directed toward solid electrolytes that can provide improved performance and are compatible with the lithium metal anode, which currently exhibits the highest theoretical specific power capacity.
Now, researchers at the University of Hong Kong (HKU) have engineered solvent-free single-ion polymer electrolytes which have delivered a significant improvement in ionic transport for lithium-metal battery applications.
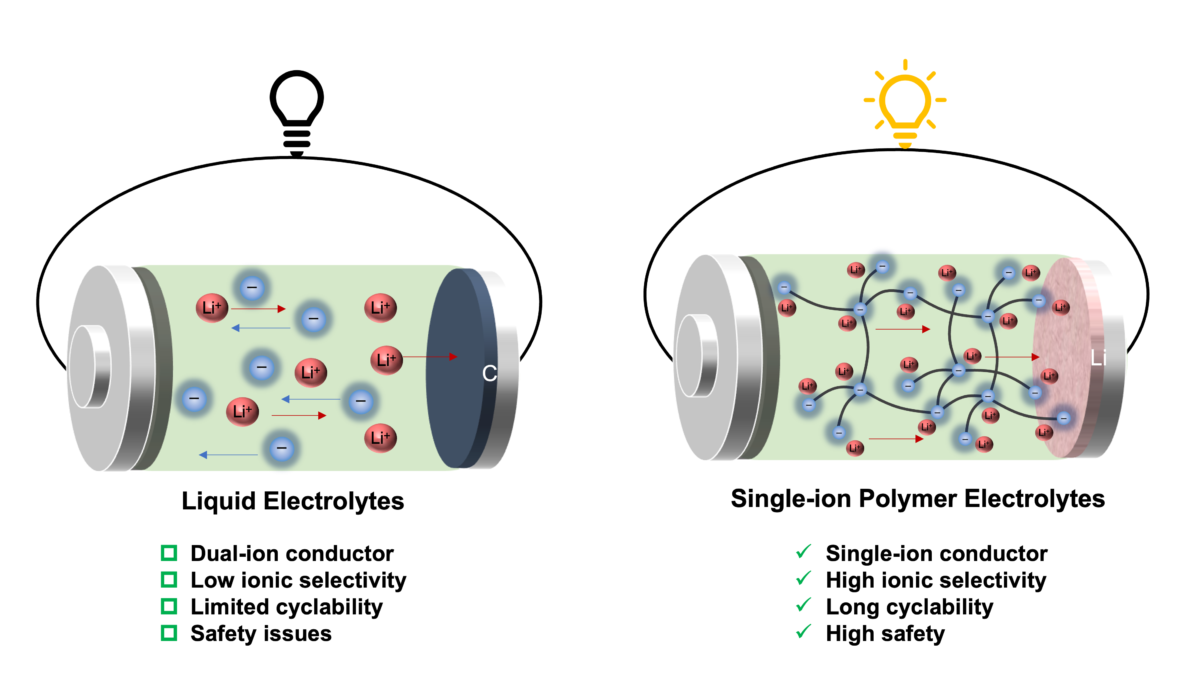
Namely, in liquid electrolytes, lithium cations and counter anions move in opposite directions to conduct electricity. Normally, anions move at least four times faster than lithium cations, and thus lithium cation transfer contributes only a small fraction (20%) of the overall ionic current. Meanwhile excessive anions accumulate at the interface between electrode and electrolyte, causing internal short circuits and capacity fade of the battery.
The newly single-ion conducting polymer electrolytes designed by HKU researchers are reported to overcome these limitations and lead to at least fourfold increase in cationic transport.
The anionic network polymers consist of borate anions bridged by branched ethylene glycol linkers of differing stoichiometric ratios, in which the anions are tethered into the polymer frame, enabling a highly selective cation transport.
The researchers controlled the cation conductivity within the polymer by systematic engineering of segmental mobility. This helped them map out the comprehensive design rules for a new class of highly conductive solid electrolytes, successfully overcoming the persistent problems of current solid electrolytes, such as low cyclability and high overpotential.
“We believe the single-ion conducting polymer electrolytes would open up the possibility of new battery chemistries that will revolutionize the field of rechargeable batteries, and offer a high level of safety, high power density, and long life cycle,” said Jingyi Gao, the first author of the research paper.
According to Dr Dong-Myeong Shin of HKU, the ion-selective electrolytes can also result in fast charging due to low overpotential. “It can allow electric vehicles to be fully charged in just the time needed for drinking a cup of coffee. This remarkable advantage will unlock a new era of a clean energy world,” he adds.
Their findings were discussed in “Engineered networking in a family of solvent-free single-ion conducting borate network polymer electrolytes for Li-metal battery applications” published in Chemical Engineering Journal.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.