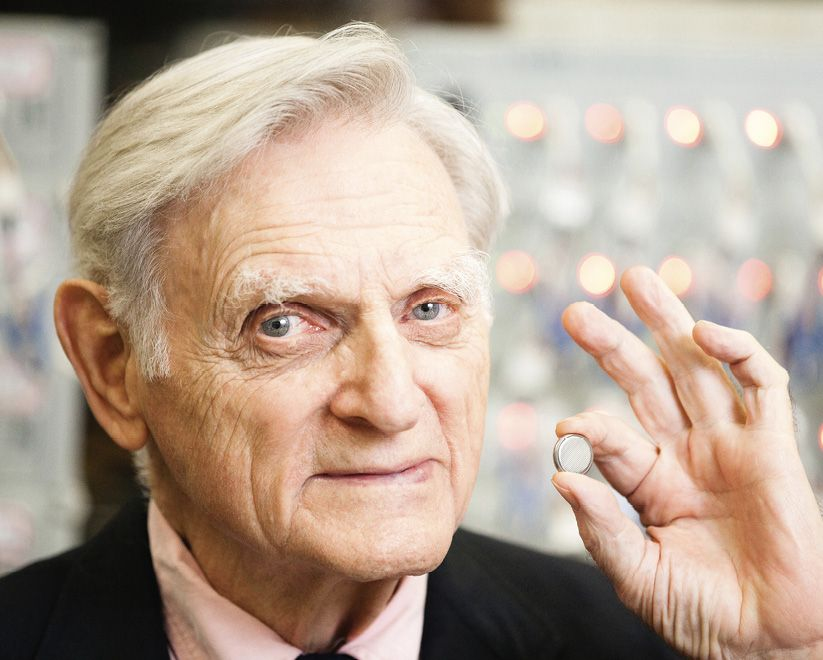
In December 2019, John Bannister Goodenough’s wheelchair was pushed center stage at Stockholm City Hall, where King Carl XVI Gustaf of Sweden was waiting. After a warm handshake and bow, the king presented Goodenough with a small red box housing his Nobel Prize in Chemistry. It recognized the 97-year-old’s crucial role in developing the rechargeable lithium-ion battery and made him the world’s oldest Nobel recipient. He died on June 25, aged 100.
Born in 1922 in Germany, Goodenough grew up in the northeastern United States. He struggled with undiagnosed dyslexia but taught himself to write and earned a boarding school scholarship. Goodenough completed his undergraduate studies in mathematics at Yale and was called to active duty in 1943 as an Army Air Force meteorologist. At the time, he became intrigued by the philosophy of science. “I was reading Whitehead’s ‘Science and the Modern World,’” he later explained. “I came to the conclusion that, if I were ever to come back from the war, and if I were to have the opportunity to go back to graduate school, I should study physics.”
Shortly after the war, federal funds enabled a group of Army officers to undertake graduate studies in the physical sciences. Unbeknownst to Goodenough, his Yale tutor had recommended him for a scholarship. In his 2008 autobiography, “Witness to Grace,” Goodenough recalled a University of Chicago (UChicago) professor who registered him saying: “I don’t understand you veterans. Don’t you know that anyone who has ever done anything significant in physics had already done it by the time he was your age?” Goodenough, who had taken only basic undergraduate courses in the subject, earned a PhD in 1952.
MIT years
While at UChicago, Goodenough met his wife, history grad student Irene Wiseman. Their shared Christian faith defined their relationship and life choices. After briefly considering ordination, Goodenough moved to MIT. He worked there for 24 years and helped lay the groundwork for computer random-access memory (RAM). While at MIT, he emerged as a founder of the modern theory of magnetism. The Goodenough-Kanamori Rules had a huge impact in telecommunication device development.
Soon after, Goodenough turned to renewables amid concern about oil trade volatility. In 1976, he was appointed head of the Inorganic Chemistry Laboratory at the University of Oxford. “I came into chemistry by the back door, working with solid-state chemists trying to build a bridge between them and the engineer and using their expertise to design experiments to explore fundamental physics questions in solid-state science,” the professor later said. That same year, oil company Exxon patented the world’s first lithium-based battery, designed by Stanley Whittingham, with whom Goodenough shared his Nobel prize. Whittingham’s battery, with lithium metal as anode and titanium sulfide as cathode, boasted low weight and large voltage capacity and operated at room temperature. It was highly unstable, however, and prone to short-circuits.
Goodenough considered how to improve Whittingham’s design and selected compounds he had studied while developing RAM: oxides. In 1980, his team achieved first demonstration of an effective rechargeable lithium battery, based on lithium cobalt oxide. Japanese chemist Akira Yoshino, the third scientist to share the Nobel prize, figured out graphite anodes made for safer, longer-lasting batteries. The resulting lithium-ion battery cell almost doubled the capacity of Whittingham’s and had low overheating risk. Sony recognized its potential. In 1991, it commercialized a battery using Goodenough’s cathode, sparking a wireless revolution toward portable electronics, lifesaving medical devices, electric cars, and more viable renewables.
Time in Texas
Oxford required Goodenough retire at 65 so, a year early, in 1986, he moved to the University of Texas (UT) at Austin. His focus remained battery research and, in 1997, he discovered another key family of cathode materials – lithium ferro-phosphate (LFP), which is today emerging as the leading stationary storage chemistry. Told by a BBC interviewer his discoveries had changed human life, Goodenough said he did not “think about it too much.”
“I’m very gratified that I’ve provided something for the people of this world,” he said. He joked he did not have a mobile phone because he didn’t like to be “bothered.”
He was known for his quick wit and infectious laugh, according to UT. “That laugh could be heard reverberating through UT engineering buildings,” the institution said. “You knew when Goodenough was on your floor and you couldn’t help but smile at the thought of running into him.” He donated his award cash prizes to graduate students and researchers. “John’s legacy as a brilliant scientist is immeasurable,” said UT Austin President Jay Hartzell. “His discoveries improved the lives of billions … He was a leader at the cutting edge of scientific research throughout the many decades of his career and he never ceased searching for innovative energy storage solutions.”
Goodenough was working well into his 90s. “Don’t retire too early” was his refrain. His final work focused on a lithium- or sodium-doped glass electrolyte to make batteries safer by eliminating flammable liquid electrolyte. “I believe the meaning of life is what we serve,” he told the Nobel Foundation in 2019. “And we have to choose very carefully because that will determine what we become. There is work to be done. So I continue.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



5 comments
By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.