It is often said that LFP batteries are safer than NMC storage systems, but recent research suggests that this is an overly simplified view.
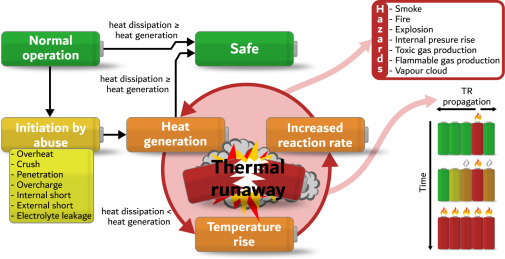
In the rare event of catastrophic failure, the off-gas from lithium-ion battery thermal runaway is known to be flammable and toxic, making it a serious safety concern. But while off-gas generation has been widely investigated, until now there has been no comprehensive review on the topic.
In a new paper, researchers from the University of Sheffield, Imperial College London, and the University of St Andrews in the United Kingdom have conducted a detailed meta-analysis of 60 papers to investigate the most influential battery parameters and the probable off-gas characteristics to determine what kind of battery would be least hazardous.
They have found that while NMC batteries release more gas than LFP, but that LFP batteries are significantly more toxic than NMC ones in absolute terms.
Toxicity varies with state of charge (SOC). Generally, a higher SOC leads to greater specific gas volume generation.
When comparing the previous findings for both chemistries, the researchers found that LFP is more toxic at lower SOC, while NMC is more toxic at higher SOC. Namely, while at higher SOC LFP is typically shown to produce less off-gas than other chemistries, at lower SOC volumes can be comparable between chemistries, but in some cases LFP can generate more.
Prismatic cells also tend to generate larger specific off-gas volumes than offer cell forms.
The composition of off-gas on average is very similar between NMC and LFP cells, but LFP batteries have greater hydrogen content, while NMC batteries have greater carbon monoxide content.
To assess the fire hazard of each chemistry, the researchers calculated and compared the lower flammability limit (LFL) of the off-gasses. They have found that LFL for LFP and NMC are 6.2% and 7.9% (in an inert atmosphere) respectively. Given the LFL and the median off-gas volumes produced, LFP cells breach the LFL in a volume 18% smaller than NMC batteries.
“Hence LFP presents a greater flammability hazard even though they show less occurrence of flames in cell thermal runaway tests,” the researchers said.
They discussed their findings in “Review of gas emissions from lithium-ion battery thermal runaway failure – Considering toxic and flammable compounds,” which was recently published in the Journal of Energy Storage.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.



The headline suggests this article is about how safe the batteries are, when actually it is about their relative toxicities once they are burning. Surely any discussion of their safety would involve the risk of them burning in the first place. Which is more likely to burn?
It seems LFP is more likely to burn according to this statement, mainly due to LFP breaching the LFL at lower volumes than NMC:
To assess the fire hazard of each chemistry, the researchers calculated and compared the lower flammability limit (LFL) of the off-gasses. They have found that LFL for LFP and NMC are 6.2% and 7.9% (in an inert atmosphere) respectively. Given the LFL and the median off-gas volumes produced, LFP cells breach the LFL in a volume 18% smaller than NMC batteries.
“Hence LFP presents a greater flammability hazard even though they show less occurrence of flames in cell thermal runaway tests,” the researchers said.
This is correct, thanks for your comment, Kirk.
This paper does not analyze the likelihood of a thermal runaway in different chemistry types but rather the relative risks and considerations in case a cell enters a thermal runaway. As a results of an analysis of TR gas emissions, it concludes that LFP batteries show a tendency for greater flammability (and the more flammable the gas is, the more likely it is to lead to explosions ) and toxicity hazards.
As per.previous comment that’s exactly what I thought…. what we need to know is which chemistry is more likely to cause a fire in normal everyday use. And I’m still of thrbmind that NMC present a significant danger which LFP do not.
I agree, which one is more likely to catch fire, under what extreme daily use, are we reliant on the electronic safty circuits, which if fail a fire will ensue??
I have six 100AH LiFePO4 Batteries. If I’m not using them, I run them all down to 70% capacity, then store them away. My top number one question is how do I protect the BMS (Battery Management system) in the event of an EMP and how do I know when the BMS has failed before something catastrophic happens? I’ve purchased an EMP Shield at EMPShield.com, but they’re expensive. So, is there a cheaper way to protect the BMS? Let me know.
The key factor is the pyrolysis temperature and for lifepo4 this is over 1100C and far in excess of that achieving a home fire. Nimc is lower and more fires have been reported with this technology.
You need what is called a Faraday Cage. Remove the BMS from the battery. Wrap it in aluminum foil completely, folding over the edges. Now a note re BMS systems:
They ALL draw some parasitic current even if only a few microamps. I lost a 1kwh 25.6 volt LFP pack 10 years ago. I didn’t realize the BMS started drawing excessive parasitic current.
This was a custom pack I had made for a project, and cost $500. Pack totally ruined. I disassembled one cell with 0.1 volts remaining. The copper anode was totally corroded, and I could see a coppery color on the cathode.
Very expensive electroplating. So a year ago I built my own pack for $80 thanks to Battery Hookup in PA. Great prices. My BMS is now connectorized, 2P8S, so 9 pins. Long storage I disconnect the BMS.
To test for parasitic draw charge battery and let it sit for a week or so. Verify that each cell is within 0.01 volt of each other. Using a digital multimeter set to current, break the connection between each wire and battery taps and measure the current. For your 100 ah pack I would like to see all currents below 0.001 Amp,
ie 1mA. So at 1 mA you will lose 1 AH in 1000 hours, or 100 AH in 100,000 hours. Each year has 8640 hours, so if each cell has less than 1 mA leakage current, you should be OK for years without having to charge. BUT I would check each cell voltage every 6 months to be sure. If I had done that I would have written off a $500 battery.
Hope this helps